Atmospheric Composition
Understanding Atmospheric Composition is both simple and handy in understanding how mankind can influence climate. Many people think the atmosphere is just too big for humans to influence? It sounds like a reasonable statement, until you realize that you don’t need to change the whole atmosphere to change climate… you just need to change a little bit of it.
With no understanding of the atmosphere and it’s composition, it is easy to see why someone might question how we could change the climate. The atmosphere is really big. But to get this in perspective, you really only need to know how much of the atmosphere keeps us warm.
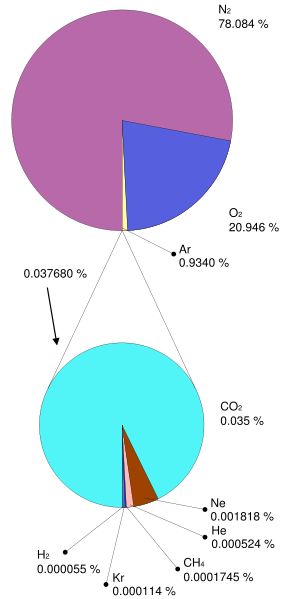
Generally speaking, looking at the pre-industrial atmosphere, if you don’t consider water vapor (a larger variable gas), the atmosphere is about 78% Nitrogen and 21% Oxygen. So, 99% of the atmosphere is accounted for in those two gases. The last one percent includes various trace gases, some are greenhouse gases, and some are not.
To get that in perspective, think about it like this. the natural greenhouse gases are CO2, H2O, CH4, and N2O. These gases comprise less than 300ths of a percent of our pre-industrial atmosphere. Then of course add water and stir 🙂
If it were not for that tiny fraction of greenhouse gases, in our atmosphere, Earth would be a giant frozen ball in space. It’s as simple as that.
Once you understand that you don’t need to change the whole atmosphere but rather you need only change a tiny fraction to change the climate or climate forcing… It becomes much easier to understand how industrial emissions can have an impact on the climate of the planet. Keep in mind that our pre-industrial atmosphere only needed, other than H2O approximately 300th’s of a percent of the atmosphere to contain GHG’s in order to keep Earth relatively warm. Changing CO2 concentration from 280ppm to approximate 400ppm is a significant increase. Now, other than H2O, nearly 400th’s of a percent of our atmosphere contains greenhouse gases. Of all the GHG’s CO2 is a more significant component because of it’s atmospheric lifetime and the associated warming ability that goes along with this particular gas, which changes the amount of heat energy trapped within our climate system.
Table 7a-1: Average estimated composition of the atmosphere up to an altitude of 25 km (*+/- 0.5%).
* considered relatively variable gases ** parts per million |
Atmospheric Composition Change: July 09, 2011
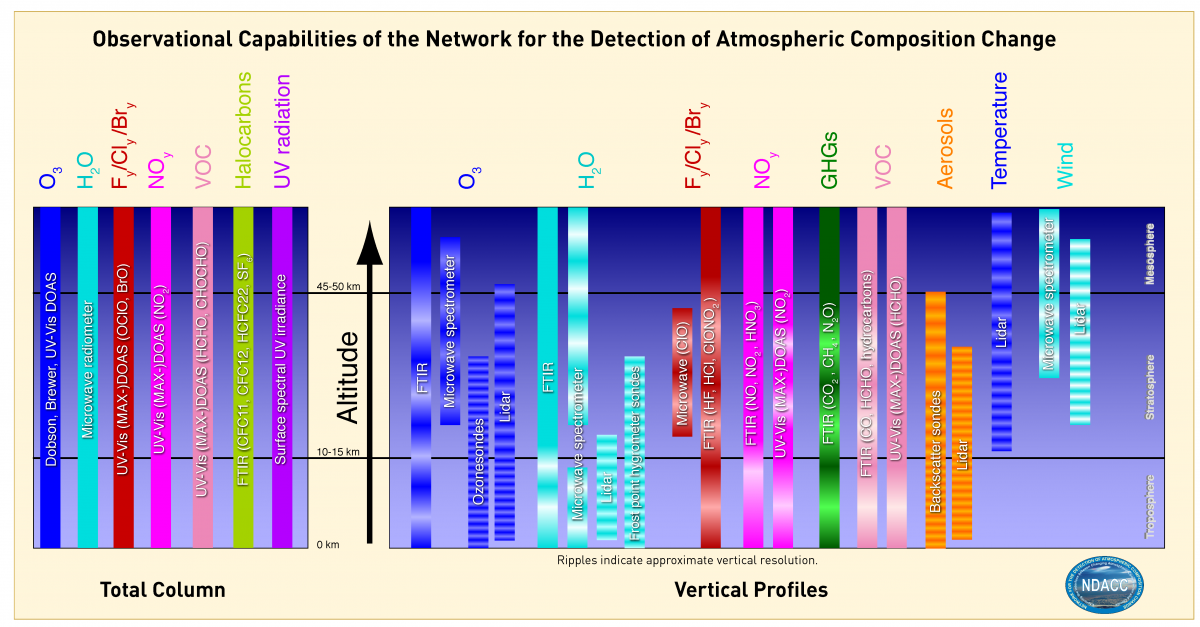
Observational Capabilities of the Network for the Detection of Atmospheric Composition Change
Source: https://ndacc.larc.nasa.gov/instruments/working-groups
Links
- https://daac.gsfc.nasa.gov/acdisc/
- https://disc.sci.gsfc.nasa.gov/acdisc/
- https://www.ndsc.ncep.noaa.gov/
- https://www.usgcrp.gov/usgcrp/ProgramElements/atmosphere.htm
- https://sedac.ciesin.columbia.edu/mva/iamcc.tg/eosdata.html
- https://www.physicalgeography.net/fundamentals/7a.html
Atmospheric Science:
- 2009 https://www.realclimate.org/index.php/archives/2009/04/yet-more-aerosols-comment-on-shindell-and-faluvegi/
- Water vapour: feedback or forcing? (
 ) (
) ( )
) - Et Tu LT?
- The tropical lapse rate quandary
- Busy Week for Water Vapor
- More satellite stuff
- Naturally trendy?
- Cloudy outlook for albedo?
- On a Weakening of the Walker Circulation
- The sky IS falling (
 ) (
) ( )
) - On Mid-latitude Storms
- Uncertainty in polar ozone depletion?
- Tropical tropospheric trends
- Butterflies, tornadoes and climate modelling
- Tropical tropospheric trends again
- Tropical tropospheric trends again (again) (
 ) (
) ( )
) - Ozone holes and cosmic rays (
 ) (
) ( )
)
Related contentKeeling Curves